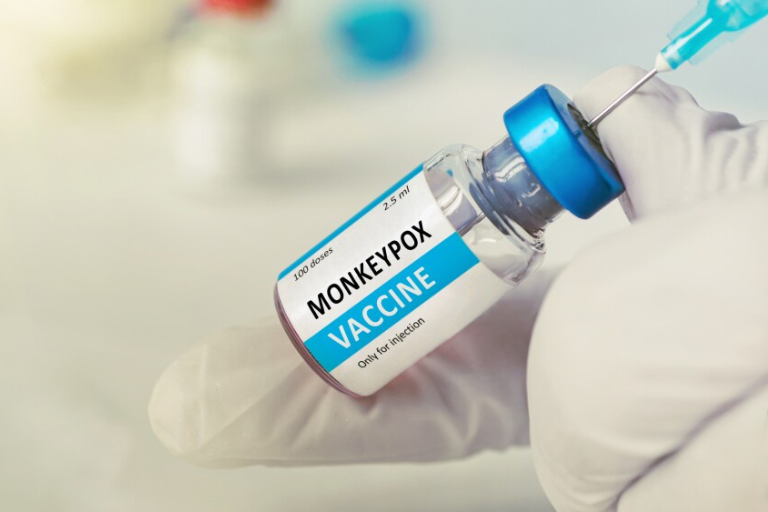
In response to ongoing global health efforts to manage mpox, the World Health Organization (WHO) has issued comprehensive guidelines and information regarding the mpox vaccine. This update is crucial for public health officials, healthcare providers, and the general public as the world continues to navigate through outbreaks of this virus.
What is Mpox?
Mpox, also known as monkeypox, is a viral zoonotic disease that primarily occurs in tropical rainforest areas of Central and West Africa but has recently spread to other regions. It is similar to smallpox but is generally less severe. The virus is transmitted to humans from animals, and human-to-human transmission occurs through close contact with lesions, body fluids, respiratory droplets, and contaminated materials.
Available Vaccines and Effectiveness
The mpox vaccine options recommended by WHO include both traditional smallpox vaccines and newer vaccines specifically targeted at mpox. Traditional vaccines, such as ACAM2000, have been shown to offer cross-protection against mpox due to the similarity between the smallpox and mpox viruses. Newer vaccines like JYNNEOS (also known as Imvamune or Imvanex) have been developed specifically for mpox and are proving to be effective.
Studies indicate that these vaccines are effective in preventing mpox. For instance, data from recent outbreaks show that individuals vaccinated with JYNNEOS had a significantly lower risk of contracting the virus compared to those who were not vaccinated. The level of protection varies depending on the individual’s health status and the timing of the vaccination.
Who Should Get Vaccinated?
WHO emphasizes the importance of vaccination for individuals at higher risk of mpox exposure. This includes healthcare workers, laboratory personnel handling specimens from suspected mpox cases, people in direct contact with infected individuals, and residents of areas experiencing outbreaks. Areas currently experiencing outbreaks include certain regions in Central and West Africa and locations with recent cases reported in Europe and North America.
Safety and Side Effects
The mpox vaccine, particularly JYNNEOS, is generally considered safe for individuals aged 18 and older. For younger age groups, especially children, the safety and efficacy of the vaccine are still being studied. Common side effects are similar to those of other vaccines and may include mild symptoms such as pain at the injection site, fever, and fatigue. Serious side effects are rare but are monitored closely by health authorities. It is always advisable to consult healthcare providers for guidance specific to individual health conditions and vaccination recommendations.
Vaccination Schedule
The mpox vaccine is administered in a series of injections. For example, JYNNEOS requires two doses given four weeks apart for optimal protection. The exact number and schedule of doses depend on the vaccine used and the specific guidelines issued by health authorities. WHO provides detailed recommendations on vaccination schedules to ensure optimal protection.
Availability and Access
Access to mpox vaccines varies globally. While some countries have ample supplies and robust vaccination programs, others, particularly in low-income regions, face challenges in vaccine availability and distribution. WHO and other global health organizations are working to ensure equitable access to vaccines for all populations, particularly those in high-risk areas.
In conclusion, the mpox vaccine is a crucial tool in managing the spread of the virus. Staying informed about vaccination recommendations and consulting healthcare providers can help individuals make the best decisions for their health.